
Levetiracetam Concentrated Solution for Injection
Belongs to Category:
The company has production licenses for tablets, capsules, granules, suppositories, powders, injections, oral solutions, dry suspensions, and eye drops.
Product Description
Approval Number:
H20203521
Indications:
It is used for the adjunctive treatment of partial seizures (with or without secondary generalized seizures) in adult and children aged 4 years and above.
This product can be used as an alternative to oral formulations when oral administration is temporarily not possible for patients.
Administration:
Levetiracetam initial treatment can be administered intravenously or orally.
Oral and intravenous administration can be directly converted without the need to gradually increase or decrease the drug dosage. The total daily dosage and the number of administrations remain unchanged.
This product can only be administered intravenously. When administering, the recommended dose of the concentrated solution should be diluted in 100 ml of diluent, and then an intravenous infusion should be carried out for 15 minutes. The diluents can be one of the following:
-0.9% Sodium Chloride Injection
-Lactated Ringer's Injection
-5% Glucose Injection
Currently, there is no clinical experience of administering this product intravenously for more than 4 consecutive days.
The usage and dosage of levetiracetam concentrated solution for injection refer to the usage and dosage of the oral formulation, as detailed below:
Adults (≥18 years old) and adolescents (12 years old - 17 years old, and weighing ≥50 kg)
The initial treatment dose is 500 mg per administration, twice a day.
According to the clinical effect and tolerance, the daily dose can be increased or decreased by 500 mg every 2 - 4 weeks, twice a day; the maximum dose is 1500 mg per administration, twice a day.
Elderly people (≥65 years old)
Adjust the dose according to the renal function status (see the description of patients with renal impairment below for details).
Children and adolescents aged 4 - 11 years old (12 - 17 years old, weighing <50 kg)
The initial treatment dose is 10 mg/kg, twice a day.
According to the clinical effect and tolerance, the dose can be increased to 30 mg/kg, twice a day. The dose change should be an increase or decrease of 10 mg/kg every 2 weeks, twice a day. The lowest effective dose should be used as much as possible.
For children weighing ≥50 kg, the dosage is the same as that for adults.
Doctors should select appropriate drug formulations and specifications according to the patient's body weight and administration dosage.
Recommended dosage for adolescents and children
| Weight | Starting dose: 10 mg/kg twice daily | Maximum dose: 30 mg/kg, 2 times a day |
| 15kg(1) | 150 mg twice daily | 450 mg twice daily |
| 20kg(1) | 200 mg 2 times daily | 600 mg twice daily |
| 25kg | 250 mg 2 times daily | 750 mg 2 times daily |
| From 50kg(2). | 500 mg 2 times daily | 1500 mg 2 times daily
|
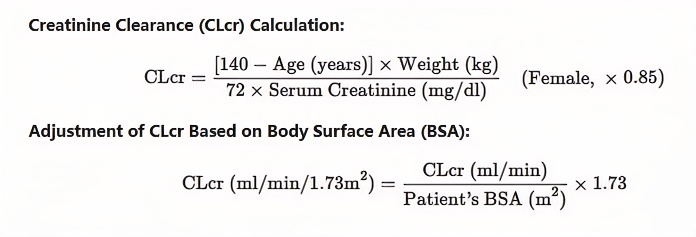 |
The adult dose is adjusted according to the patient's renal function status
| Patient group | Creatinine Clearance Rate | Dosage and number of doses |
| Normal patients | ≥80 | 500-1500 mg 2 times a day |
| Mild abnormalities | 50-79 | 500-1000 mg 2 times a day |
| Moderately abnormal | 30-49 | 250-750 mg 2 times daily |
| Severe abnormality | <30 | 250-500 mg 2 times a day |
| Patients with end-stage renal disease undergoing dialysis(1). | - | 500-1000 mg once daily(2). |
(1)The recommended loading dose on the first day of taking is levetiracetam 750mg.
(2)After dialysis, a supplementary dose of 250-500 mg is recommended.
Pediatric patients with renal impairment should adjust the dose according to renal functional status, as clearance of levetiracetam is related to renal function. However, they are based on studies in adults with renal impairment.
Creatinine clearance CLcr (ml/min/1.73m2) is estimated by measuring serum creatinine (mg/dl) values, and creatinine clearance in adolescent and pediatric patients can be obtained by the following formula:
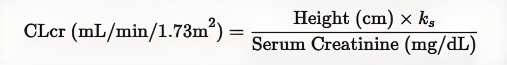
KS = 0.55 (children under 13 years of age); KS = 0.7 (male adolescent)
| Patient group | Creatinine Clearance Rate (ml/min/1.73m2) | Dosage and number of doses |
| Normal patients | >80 | 10 to 30 mg/kg (0.10 to 0.30 ml/kg) twice daily |
| Mild abnormalities | 50-79 | 10 to 20 mg/kg (0.10 to 0.20 ml/kg) twice daily |
| Moderately abnormal | 30-49 | 5 to 15 mg/kg (0.05 to 0.15 ml/kg) twice daily |
| Severe abnormality | <30 | 5 to 10 mg/kg (0.05 to 0.10 ml/kg) twice daily |
| Patients with advanced renal disease who are on dialysis | - | 10-20mg/kg (0.10-0.20ml/kg) once daily(1) (2) |
- On the first day of administration, the recommended loading dose of levetiracetam is 15 mg/kg (0.15 ml/kg).
(2) After dialysis, an additional dose of 5-10 mg/kg (0.05-0.10 ml/kg) is recommended.
For patients with liver diseases
For patients with mild and moderate liver function impairment, there is no need to adjust the dosage. For patients with severe liver impairment, calculation based on creatinine clearance may underestimate the degree of renal function impairment. Therefore, if the patient's creatinine clearance is less than 60 ml/min/1.73 m², the daily dose should be halved.
Specifications:
5ml: 500mg
The company has production licenses for tablets, capsules, granules, suppositories, powders, injections, oral solutions, dry suspensions, and eye drops.
The main products cover cardiovascular diseases, diabetic complications, anti-tumor, calcium supplementation, digestive system, respiratory system, and orthopedics.
Related Products
Online Message
We will contact you within one business day. Please pay attention to your email.
Email: marcos.yuan@wangao.com.cn
Address: No. 688, Dinghai Road, Haimen City, Jiangsu Province

Mobile Version QR Code

Whatsapp Account
Copyright © 2025 Jiangsu Wangao Pharmaceutical Co., Ltd.







